Plastic Injection Molding for Airway Management Devices and Anesthesia Equipment
PreciKam supports medical device manufacturers in the production of injection-molded plastic components for airway management devices used to facilitate intubation in surgical and emergency care. As your single-source partner, we provide ISO Class 7 and ISO Class 8 clean room injection molding services as well as assembly and packaging to limit the number of hands involved in your project. With decades of experience and ISO 13485:2016 certified, you can rely on us to deliver the precision, traceability, and strict quality standards your application requires, from first prototypes to final production.
Reasons to Choose PreciKam for Airway Management Device Manufacturing
At PreciKam, we understand that when lives are at stake, there’s no room for compromise. Medical device developers and manufacturers can rely on us to produce their critical parts with precision, consistency, and care. Our approach is based on risk management and in-depth collaboration, making us the ideal partner for the manufacture of airway and anesthetic devices.

Reasons to Choose PreciKam for Airway Management Device Manufacturing
At PreciKam, we understand that when lives are at stake, there’s no room for compromise. Medical device developers and manufacturers can rely on us to produce their critical parts with precision, consistency, and care. Our approach is based on risk management and in-depth collaboration, making us the ideal partner for the manufacture of airway and anesthetic devices.
By choosing PreciKam, you gain access to:

Reasons to Choose PreciKam for Airway Management Device Manufacturing
At PreciKam, we understand that when lives are at stake, there’s no room for compromise. Medical device developers and manufacturers can rely on us to produce their critical parts with precision, consistency, and care. Our approach is based on risk management and in-depth collaboration, making us the ideal partner for the manufacture of airway and anesthetic devices.
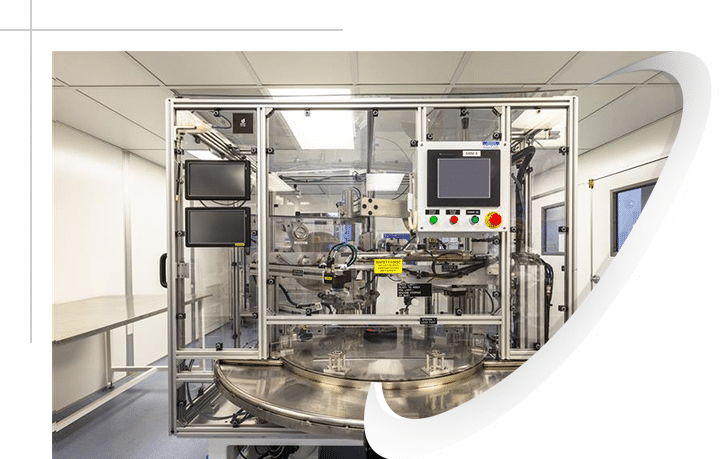
Our Capabilities for Airway Management Devices and Anesthesia Equipment Manufacturing
PreciKam has developed expertise in the manufacture of medical devices that require tight tolerances, strict cleanliness requirements, and attention to detail. As a custom precision plastic injection molder, we’ll work with you to develop and produce your next-gen airway management devices and components to your specific requirements and standards. Choosing Precikam means having access to the flexibility and knowledge that other larger molders can’t necessarily provide.
Precision Molding
Tight tolerance capabilities down to ± 0.01 mm using medical-grade certified resins from trusted suppliers.
Multi-Part Assemblies
Complex assemblies up to 12-13 components built under rigorous quality management systems.

Advanced Technologies
Overmolding and insert molding for hybrid designs, plus specialized printing on plastic for instructions and warnings, including UV printing
Complete Traceability
Full process and documentation validation (IQ, OQ, PQ) and complete traceability for quality and regulatory compliance
Project Collaboration & Flexibility
PreciKam P5™: From Prototype to Production
Every project at PreciKam follows our structured P5 process—Program Definition, Part Design, Process Development, Production, and Performance Monitoring. This approach ensures we align with your goals from the outset and maintain rigorous control over quality and timelines.
You are involved at every step—from prototyping to production—ensuring a transparent process and a reliable outcome.


Project Collaboration & Flexibility
From Prototype to Production
Every project at PreciKam follows our structured P5 process—Program Definition, Part Design, Process Development, Production, and Performance Monitoring. This approach ensures we align with your goals from the outset and maintain rigorous control over quality and timelines.
You are involved at every step—from prototyping to production—ensuring a transparent process and a reliable outcome.

Project Collaboration & Flexibility
From Prototype to Production
Every project at PreciKam follows our structured P5 process—Program Definition, Part Design, Process Development, Production, and Performance Monitoring. This approach ensures we align with your goals from the outset and maintain rigorous control over quality and timelines.
You are involved at every step—from prototyping to production—ensuring a transparent process and a reliable outcome.


